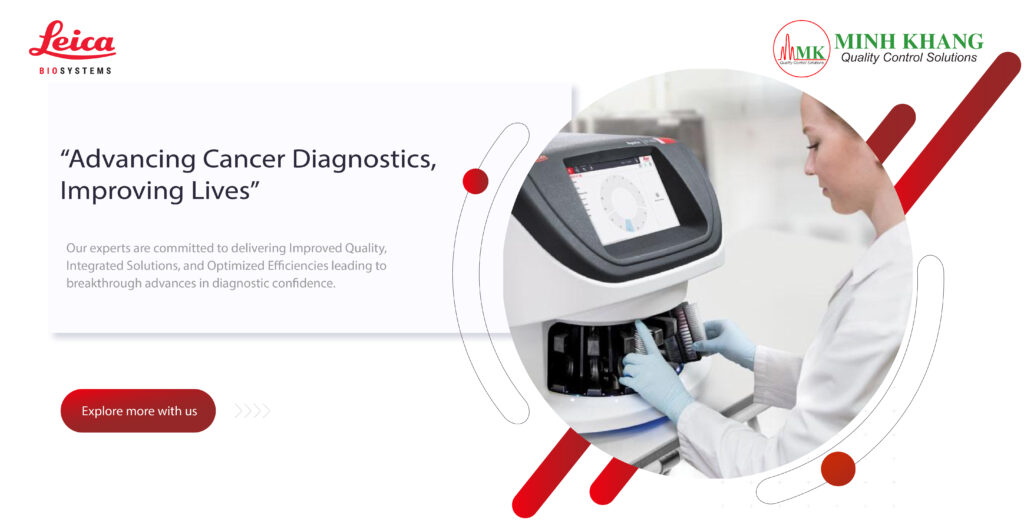
Applications
- Small volume injectable (SVI)
- Large volume injectable (LVI)
- Research & development
- Troubleshooting
- Stability testing
Sample management
- Instant particle contamination notification
- Small vial holder & tare volume conserves costly samples
- Configurable flow rates & interchangeable sensors for 0.5 µm – 600 µm particle size detection
- Interchangeable sample probes & syringes
Software
- Program custom SOPs to eliminate manual counter configuration and pass/fail calculations
- Multi-level security for 21 CFR Part 11 compliance
- Automatic database backup & recovery loss feature protects valuable results and data
Support
- Expert SOP & IQ/OQ validation services available
- Routine onsite service available for maximum uptime
- ISO 21501 compliant calibrations


















 VI
VI