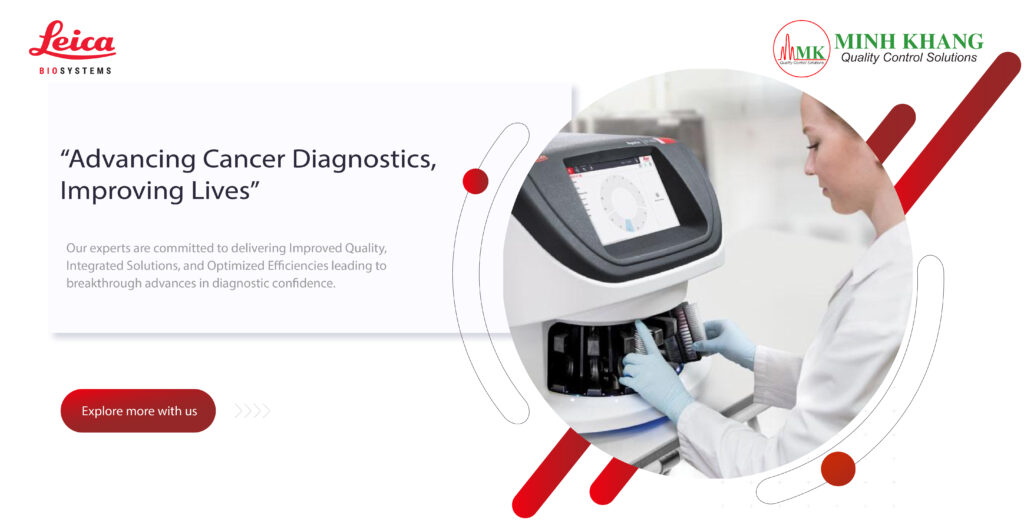
DIS 800i is a reliable and cost-efficient dissolution tester system designed with the highest standards of solid dosage testing performance in mind. Designed to minimise user training and reduce the burden of routine equipment maintenance, the DISi dissolution tester series from Copley simplifies the dissolution testing process, without compromising on data quality.
Highlight:
- Ph. Eur. and USP compliant
- Integrated, precision temperature control and measurement
- Intuitive touchscreen control to simplify operation
- Single-point electronic temperature calibration
- Eight test station unit configurations available
- Extensive data reporting output options: RS 232, USB A and USB B
- Wide speed range to accommodate broad scope of methods
- Option to automate and remotely control DISi Series systems
















 VI
VI