INTRODUCTION
Various pharmacopoeias, including the USP, specify a test for mycoplasma contamination must be performed as part of the release testing of a product manufactured in the presence of eukaryotic cells (1).
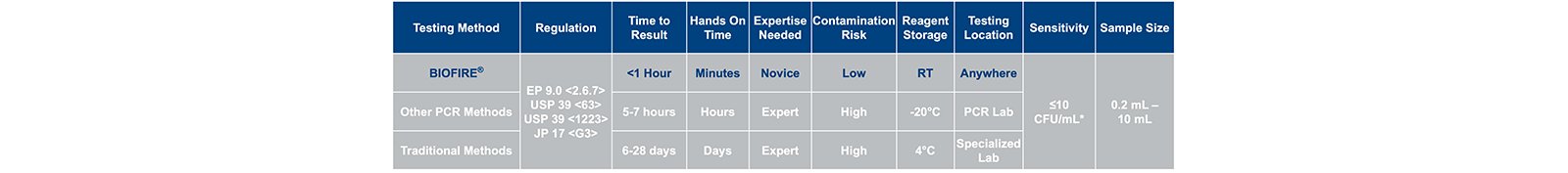
Current compendial methods require ≥28 days to generate results creating delays releasing product to downstream processes. Alternative conventional nucleic acid testing (NAT) methods are available; however, until recently these methods provided speed (~5 h) but not ease of use.
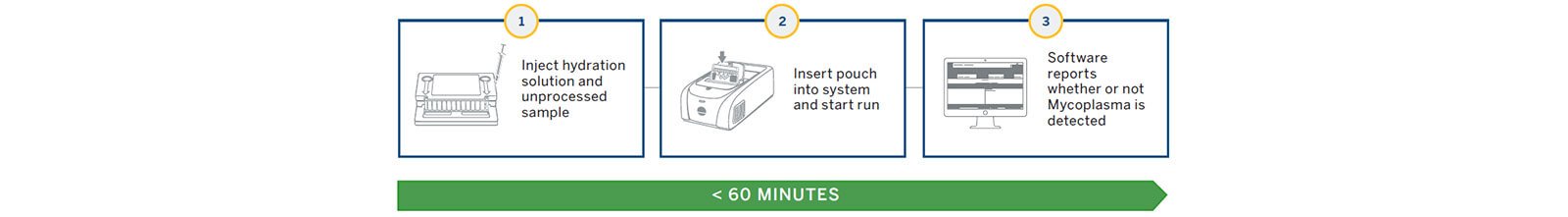
The BIOFIRE® FILMARRAY® 2.0 industry system utilizes a film array instrument and next generation PCR testing where all reagent components are contained in a closed pouch to detect the presence of >130 species of mycoplasma. The system provides sample to answer in ~1 hour with little technical training needed providing options for bringing mycoplasma testing directly to the production floor where critical testing is needed.
*10 mL protocol
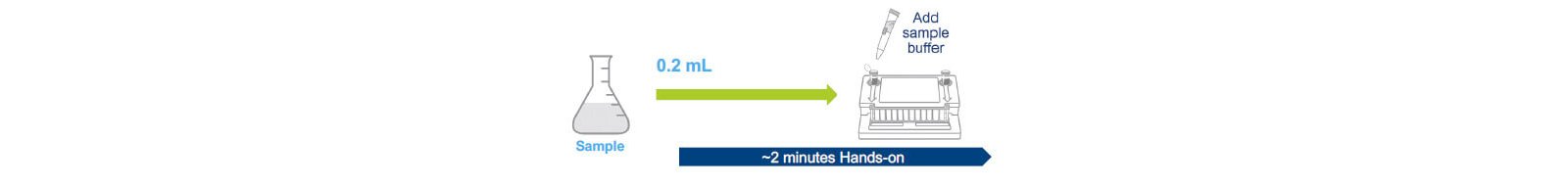
As presented at the 2021 PDA Pharmaceutical Microbiology Virtual Conference, the data summarized in this White Paper is from four bioproduction manufacturers that evaluated the BIOFIRE®FILMARRAY® 2.0 Industry system (2). Samples were evaluated with up to 9 compendial mycoplasma species in the presence of high-density monoclonal antibody producing Chinese hamster ovary (CHO) cells. Studies were designed to assess product interference (false-positive rates), and detection (false-negative rates) including level of detection (LOD). Two distinct protocols were evaluated: a protocol using 10 mL of product sample that provides the sensitivity for release testing, and a direct-test protocol using 0.2 mL product sample that allows for at-line in-process control testing.
THE VALUE OF RAPID, EASY MYCOPLASMA TESTING
A faster, easier approach to mycoplasma testing can bring quantifiable value to bioproduction manufacturers. From simplifying training requirements, to reducing investigations linked to human error, to providing an early alert to contamination, an ultra rapid, ultra easy mycoplasma test can save time and money bringing value to any bioproduction organization.
The BIOFIRE® mycoplasma test can be performed by anyone, anywhere at anytime allowing manufacturers to take critical quality testing out of the lab and closer to manufacturing to realize the following benefits:
• Simplified training requirements
• Lower expertise required
• Objective results
• Less human error risk/improved Data Integrity
• Does not require PCR lab
• 1 hour time-to-result brings flexibility in testing planning
• Provides early alert to contamination and reduces costs of non-quality

Because BIOFIRE® FILMARRAY® 2.0 is so easy to use, manufacturers who previously lacked the resources to perform mycoplasma testing in-house can now easily perform mycoplasma screening at-line with little specialized equipment or training saving on costly outsourced laboratory testing.
Bioproduction manufacturers today waiting hours or weeks for results using complex testing methods can realize significant value with a more rapid, simplified approach to mycoplasma testing using BIOFIRE®FILMARRAY® 2.0 Industry.
BIOFIRE FILMARRAY 2.0 INDUSTRY SYSTEM
The system utilizes a FILMARRAY® 2.0 Industry instrument and next generation PCR testing in a closed pouch to detect the presence of mycoplasma (Figures 1 and 2). The disposable BIOFIRE® Mycoplasma pouch contains all of the necessary reagents for automated cell lysis, nucleic acid purification, reverse transcription, first and second stage nested PCR and analyst detection in order to isolate, amplify and detect over 130 different mycoplasma species (Figure 2).
Several controls are integrated into the pouch to ensure the quality of the results including a total process control, reverse transcription control, and PCR I and PCR II controls. The instrument and software process the pouch with results in less than an hour.
The FILMARRAY® 2.0 Industry software (21 CFR Part 11 compliance ready) performs all of the complex meta-analysis and provides presence/absence results as either “Mycoplasma Detected” or “Mycoplasma Not Detected”.
Figure 1:
FILMARRAY® 2.0 Industry instrument performs the extraction, amplification and detection (25.4 x 39.3 x 16.5 cm WxDxH). The system comes standard with 2 instruments; up to 8 instruments can be connected to a single PC.
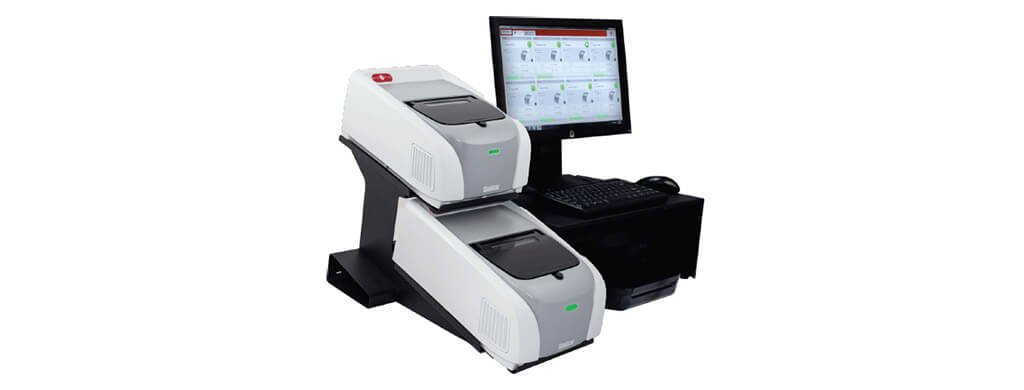
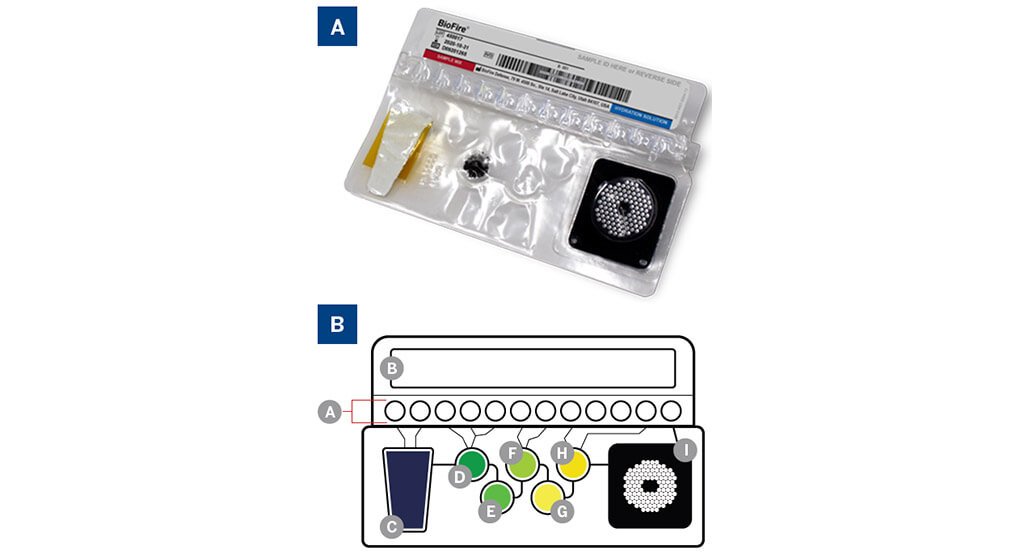
Figure 2:
A. BIOFIRE® Mycoplasma pouch.
B. Pouch diagram: (A) Fitment with freezedried reagents; (B) Plungers-deliver reagents to blisters; (C) Sample lysis and bead collection; (D) Wash; (E) Magnetic bead collection blister; (F) Elution; (G) Multiplex Outer PCR blister; (H) Dilution blister; (I) Inner Nested PCR array
SAMPLE PROTOCOLS
Two distinct protocols have been designed to detect mycoplasma contamination(3). These include a 0.2 mL direct test that can be used for in-process control testing with a validated LOD of ~≤30 CFU/ mL, and a 10 mL release test that concentrates the sample using centrifugation and has a validated LOD of ≤10 CFU/mL.
These protocols were followed by evaluators except that Evaluator B deviated from the manufacturer recommendations with customized centrifugation times and forces. Following sample pre-processing, samples were then loaded onto a fully prepared and hydrated pouch and run on the FILMARRAY® 2.0 Industry instrument.


 VI
VI